PROVEN EXPERIENCE
Unparalleled clinical experience, with a well-established safety and tolerability profile1,8-12
This site is based on the EU Summary of Product Characteristics, and is
intended for healthcare professionals outside of the US, UK, and Ireland.
Hereditary angioedema (HAE) is a rare genetic disease that causes recurrent attacks of angioedema in the body, including the hands, feet, genitals, gastrointestinal tract, face—and larynx, where attacks can be life-threatening.2,3
The frequency, severity, and location of previous attacks are not predictors of future HAE attacks2—approximately 50% of patients experience at least 1 laryngeal attack in their lifetimes4*
The unexpected nature of HAE can lead to psychosocial burdens and long-term lifestyle modifications, including5:
The 2021 HAE WAO/EAACI Guideline recommends evaluating patients with HAE Type I or II for preventive treatment at every visit.7
*Based on 123 patients in a German angioedema outpatient service from 1973 to 2001.4
†Based on qualitative (n=30) and quantitative (n=186) survey responses in the 2011 Hereditary Angioedema Burden of Illness Study in Europe.5,6
EAACI=European Academy of Allergy and Clinical Immunology; WAO=World Allergy Organization.

TAKHZYRO is indicated for routine prevention of recurrent attacks of hereditary angioedema in patients aged 12 years and older.1

Unparalleled clinical experience, with a well-established safety and tolerability profile1,8-12

Freedom from attacks for an average of >1 year and consistent long-term attack reduction1,8
Reduced treatment burden for patients and freedom to adjust dosage based on response1†
TAKHZYRO demonstrated significant and clinically meaningful improvement in quality of life, with some patients experiencing early and long-term improvement in functioning, fear and shame, fatigue, and nutrition.8,13,14
*Patients taking TAKHZYRO in a 6.5-month study and a 2.5-year open-label extension study had substantial reductions in attack frequency and severity. Some patients in the studies had zero attacks for periods of time.1,8,15
†The recommended starting dose is TAKHZYRO 300 mg every 2 weeks. In patients who are stably attack free on treatment, a dose reduction of TAKHZYRO 300 mg every 4 weeks may be considered, especially in patients with low weight.1

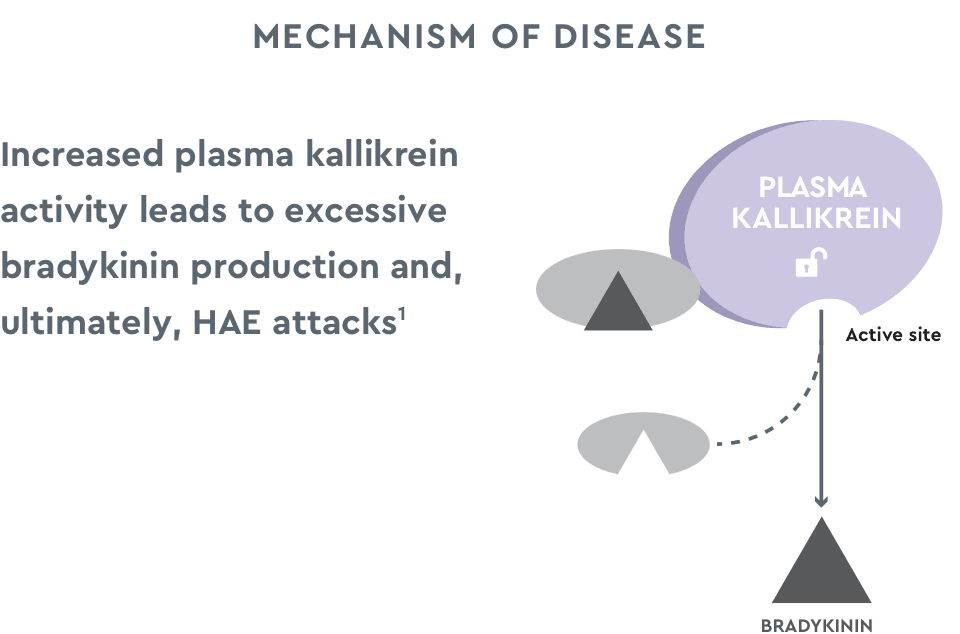
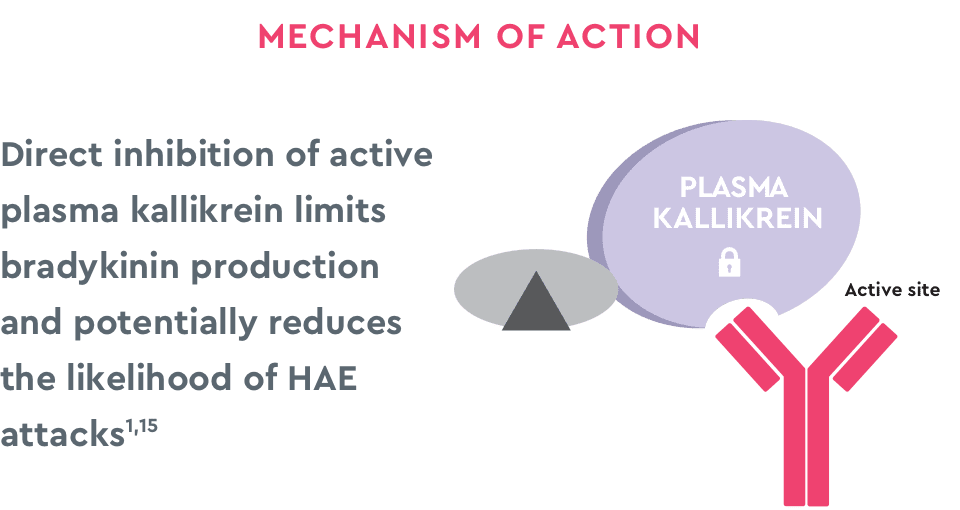

cHMWK=cleaved high molecular weight kininogen; HMWK=high molecular weight kininogen.


TAKHZYRO demonstrated statistical significance compared with placebo for all secondary efficacy endpoints in the HELP study (P<0.001).1
All results were with TAKHZYRO 300 mg every 2 weeks.
*LS mean monthly attack rate: investigator-confirmed attacks/4 weeks.1,15
†For rollover patients, the baseline attack rate was calculated as the number of investigator-confirmed HAE attacks during the run-in period of the HELP study. For nonrollover patients, the baseline attack rate was calculated as the number of HAE attacks during the historical reporting period of the last 3 months, divided by the number of days they contributed to these periods, multiplied by 28 days.8
HELP=Hereditary angioEdema Long-term Prophylaxis; LS=least squares; OLE=open-label extension.

ATTACKS
after 6 doses
After 6 doses, 77% of patients taking TAKHZYRO (n=26) had ZERO ATTACKS for 4 months vs 3% of patients taking placebo (n=37; Day 70-182; anticipated time to reach steady-state concentration is ~70 days) (post hoc sensitivity analysis).1,15*
Patients had ZERO ATTACKS on average for nearly 15 months† (415 days, mean [SD] of 14.8 [12.4] months) in the HELP OLE (N=209) (prespecified exploratory endpoint).1,8*
On average

ATTACKS
for >1 year
All results were with TAKHZYRO 300 mg every 2 weeks.
*These findings are exploratory or post hoc in nature and therefore require further investigation to corroborate.
†One month was defined as 28 days.8
HELP=Hereditary angioEdema Long-term Prophylaxis; OLE=open-label extension.
HELP=Hereditary angioEdema Long-term Prophylaxis; OLE=open-label extension.

The most common adverse reactions (ARs) were injection site reactions1,15
Zero treatment-related serious adverse events (AEs) were identified8
All results were with TAKHZYRO 300 mg every 2 weeks unless otherwise indicated.
*The patient had a history of metabolic syndrome and fatty liver and used multiple concomitant suspect medications. The patient withdrew due to isolated, asymptomatic, and transient elevation of alanine transaminase (140 U/L) and aspartate transaminase (143 U/L) classified as related and severe on Day 139.16
†Hypersensitivity reactions were diarrhoea and localised maculopapular rash; rash at the injection site and slight swelling under the eyes; and oedema, wheals, and joint pain.17
HELP=Hereditary angioEdema Long-term Prophylaxis; OLE=open-label extension.




All results were with TAKHZYRO 300 mg every 2 weeks.
GI=gastrointestinal.


*In clinical studies, the majority of patients self-administered TAKHZYRO within 10 to 60 seconds. These injection times are based on vial administration.18
This medicinal product should only be taken if prescribed. Treatment should be initiated under the supervision of a physician experienced in the management of patients with HAE.1


81%
More than 8 out of 10 patients taking TAKHZYRO experienced clinically meaningful improvement in quality of life vs 37% of patients taking placebo at 6.5 months (P<0.05).1,13,15
As shown on the Angioedema Quality of Life Questionnaire (AE-QoL), which measures the patient-reported impact of angioedema over a 4-week recall period across 4 domains, patients taking TAKHZYRO in the HELP study reported clinically meaningful improvement (reduction of ≥6) across all domains.1,14†
All results were with TAKHZYRO 300 mg every 2 weeks.
A reduction in score shows improvement.1
*These findings are tertiary endpoint analyses and therefore require further investigation to corroborate.
†Domains included functioning, fear and shame, fatigue, and nutrition.13
HELP=Hereditary angioEdema Long-term Prophylaxis.
The majority of the improvements in AE-QoL scores were observed early, with marked improvements from Day 0 to Day 56, and were generally maintained until the end of the study at 132 weeks, or ~2.5 years.21
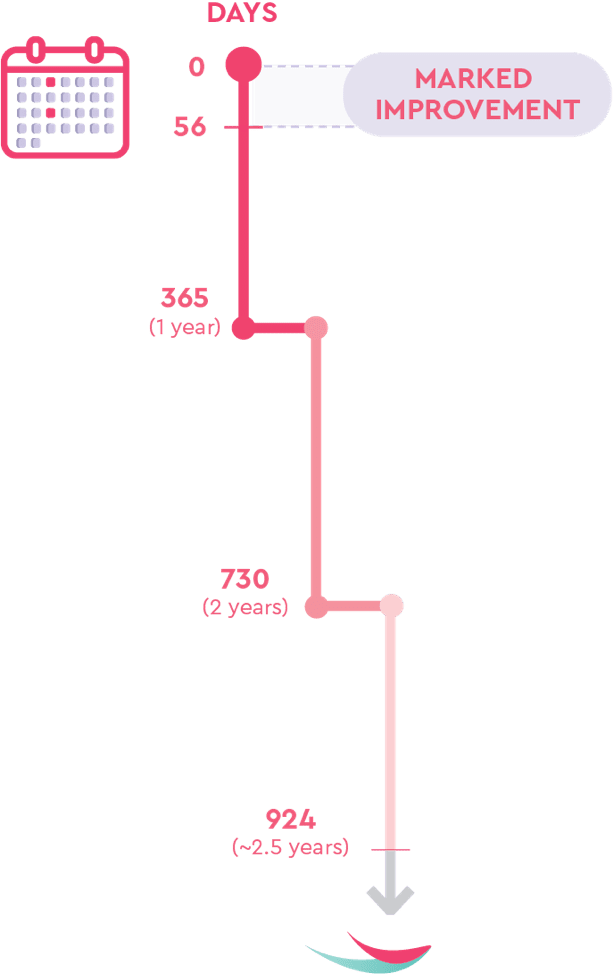
All results were with TAKHZYRO 300 mg every 2 weeks.
HELP=Hereditary angioEdema Long-term Prophylaxis; OLE=open-label extension.
The greatest improvements in the HELP study and HELP OLE were8,13,14:
All results were with TAKHZYRO 300 mg every 2 weeks.
HELP=Hereditary angioEdema Long-term Prophylaxis; OLE=open-label extension.
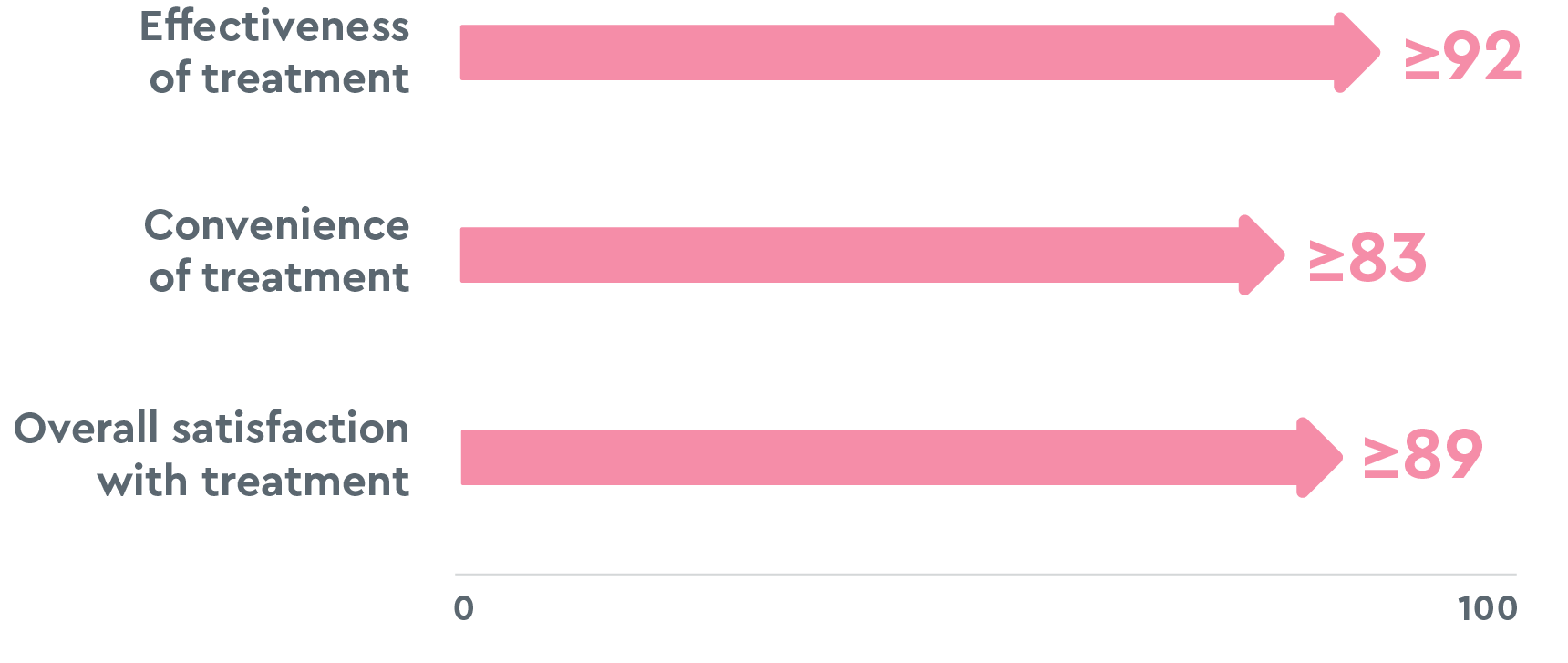
Ratings are on a scale of 0 to 100, with 100 being extremely satisfied, and are based on the end-of-the study visit.22
All results were with TAKHZYRO 300 mg every 2 weeks.
*In clinical studies, the majority of patients self-administered TAKHZYRO within 10 to 60 seconds. These injection times are based on vial administration.18
HELP=Hereditary angioEdema Long-term Prophylaxis; OLE=open-label extension.

TAKHZYRO is strongly recommended* as a first-line long-term preventive treatment option for patients 12 years of age and older by the 2021 HAE WAO/EAACI Guideline.1,7
*"Strongly recommended" means that all or almost all informed people would make that choice, that less time is needed for healthcare providers to make decisions, and that, in most clinical situations, the recommendation may be adopted as policy. In the guideline, 89% of members in the expert panel agreed with the use of TAKHZYRO as first-line long-term prophylaxis, giving TAKHZYRO a strong recommendation.7
EAACI=European Academy of Allergy and Clinical Immunology; LTP=long-term prophylaxis; WAO=World Allergy Organization.
Please consult the TAKHZYRO Summary of Product Characteristics (SmPC) before prescribing.
TAKHZYRO treatment should be initiated under the supervision of a physician experienced in the management of patients with hereditary angioedema (HAE). TAKHZYRO may be self-administered or administered by a caregiver only after training on subcutaneous (SC) injection technique by a healthcare professional.
Hypersensitivity to the active substance or to any of the excipients.
Hypersensitivity reactions have been observed. In case of a severe hypersensitivity reaction, administration of TAKHZYRO must be stopped immediately and appropriate treatment must be initiated.
Traceability: In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded.
Hypersensitivity reactions have been observed. In case of a severe hypersensitivity reaction, administration of TAKHZYRO must be stopped immediately and appropriate treatment must be initiated.
General: TAKHZYRO is not intended for treatment of acute HAE attacks. In case of a breakthrough HAE attack, individualized treatment should be initiated with an approved rescue medication. There are no available clinical data on the use of lanadelumab in HAE patients with normal C1-INH activity.
Interference with coagulation test: Lanadelumab can increase activated partial thromboplastin time (aPTT) due to an interaction of lanadelumab with the aPTT assay. The reagents used in the aPTT laboratory test initiate intrinsic coagulation through the activation of plasma kallikrein in the contact system. Inhibition of plasma kallikrein by lanadelumab can increase aPTT in this assay. None of the increases in aPTT in patients treated with TAKHZYRO were associated with abnormal bleeding adverse events. There were no differences in international normalised ratio (INR) between treatment groups.
Sodium content: This medicinal product contains less than 1 mmol sodium (23 mg) per vial, that is to say essentially 'sodium-free'.
No dedicated drug-drug interaction studies have been conducted. Based on the characteristics of lanadelumab, no pharmacokinetic interactions with co-administered medicinal products is expected.
As expected, concomitant use of the rescue medication C1 esterase inhibitor results in an additive effect on lanadelumab-cHMWK response based on the mechanism of action (MOA) of lanadelumab and C1 esterase inhibitor
Treatment with lanadelumab has been associated with development of treatment emergent anti-drug antibodies (ADA) in 11.9% (10/84) of subjects. All antibody titres were low. The ADA response was transient in 20% (2/10) of ADA positive subjects. 2.4% (2/84) of lanadelumab-treated subjects tested positive for neutralising antibodies.
The development of ADA including neutralising antibodies against TAKHZYRO did not appear to adversely affect the pharmacokinetic (PK) and pharmacodynamics (PD) profiles or clinical response.
The most commonly observed adverse reaction (52.4%) associated with TAKHZYRO was injection site reactions (ISR) including injection site pain, injection site erythema and injection site bruising. Of these ISRs, 97% were of mild intensity, 90% resolved within 1 day after onset with a median duration of 6 minutes.
Hypersensitivity reaction (mild and moderate pruritus, discomfort and tingling of tongue) was observed (1.2%)
| Very common (frequency ≥1/10): |
Injection site reactions* |
| Common (≥1/100 to <1/10): |
Hypersensitivity**, dizziness, rash maculo-papular, myalgia, alanine aminotransferase increased, aspartate aminotransferase increased. |
*Injection site reactions include: pain, erythema, bruising, discomfort, haematoma, haemorrhage, pruritus, swelling, induration, paraesthesia, reaction, warmth, oedema and rash.
**Hypersensitivity includes: pruritus, discomfort and tingling of tongue.

Suspected adverse reactions should be reported to Takeda at: GPSE@takeda.com.
Please see full EU Summary of Product Characteristics (SmPC).
References
1. Takhzyro. Summary of product characteristics. Takeda Pharmaceuticals International AG Ireland Branch; 2022. 2. Kaplan AP. Enzymatic pathways in the pathogenesis of hereditary angioedema: the role of C1 inhibitor therapy. J Allergy Clin Immunol. 2010;126(5):918-925. doi:10.1016/j.jaci.2010.08.012 3. Banerji A, Busse P, Christiansen SC, et al. Current state of hereditary angioedema management: a patient survey. Allergy Asthma Proc. 2015;36(3):213-217. doi:10.2500/aap.2015.36.3824 4. Bork K, Hardt J, Schicketanz KH, Ressel N. Clinical studies of sudden upper airway obstruction in patients with hereditary angioedema due to C1 esterase inhibitor deficiency. Arch Intern Med. 2003;163(10):1229-1235. doi:10.1001/archinte.163.10.1229 5. Bygum A, Aygören-Pürsün E, Beusterien K, et al. Burden of illness in hereditary angioedema: a conceptual model. Acta Derm Venereol. 2015;95(6):706-710. doi:10.2340/00015555-2014 6. Caballero T, Aygören-Pürsün E, Bygum A, et al. The humanistic burden of hereditary angioedema: results from the Burden of Illness Study in Europe. Allergy Asthma Proc. 2014;35(1):47-53. doi:10.2500/aap.2013.34.3685 7. Maurer M, Magerl M, Betschel S, et al. The international WAO/EAACI guideline for the management of hereditary angioedema—the 2021 revision and update. Allergy. 2022;77(7):1961-1990. doi:10.1111/all.15214 8. Banerji A, Bernstein JA, Johnston DT, et al; HELP OLE Investigators. Long-term prevention of hereditary angioedema attacks with lanadelumab: the HELP OLE study. Allergy. 2022;77(3):979-990. doi:10.1111/all.15011 9. Cinryze. Prescribing information. Takeda Pharmaceuticals USA, Inc; 2021. 10. Craig T, Zuraw B, Longhurst H, et al. Long-term outcomes with subcutaneous C1-inhibitor replacement therapy for prevention of hereditary angioedema attacks. J Allergy Clin Immunol Pract. 2019;7(6):1793-1802.e2. doi:10.1016/j.jaip.2019.01.054 11. A long term safety study of BCX7353 in hereditary angioedema (APeX-S). ClinicalTrials.gov identifier: NCT03472040. Updated January 13, 2023. Accessed February 16, 2023. https://clinicaltrials.gov/ct2/show/NCT03472040 12. Orladeyo. Prescribing information. BioCryst Pharmaceuticals, Inc; 2022. 13. Lumry WR, Weller K, Magerl M, et al; HELP Study Investigators. Impact of lanadelumab on health-related quality of life in patients with hereditary angioedema in the HELP study. Allergy. 2021;76(4):1188-1198. doi:10.1111/all.14680 14. Weller K, Groffik A, Magerl M, et al. Development and construct validation of the angioedema quality of life questionnaire. Allergy. 2012;67(10):1289-1298. doi:10.1111/all.12007 15. Banerji A, Riedl MA, Bernstein JA, et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018;320(20):2108-2121. doi:10.1001/jama.2018.16773 16. Banerji A, Riedl MA, Bernstein JA, et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. Supplement 2. Supplementary online content. JAMA. 2018;320(20):2108-2121. Accessed February 16, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6583584/bin/jama-320-2108-s002.pdf 17. Banerji A, Bernstein JA, Johnston DT, et al; HELP OLE Investigators. Long-term prevention of hereditary angioedema attacks with lanadelumab: the HELP OLE study. Supporting information. Tables S1-S3. Allergy. 2022;77(3):979-990. Accessed February 16, 2023. https://onlinelibrary.wiley.com/doi/10.1111/all.15011 18. Takhzyro. Prescribing information. Dyax Corp; 2023. 19. Cinryze. Summary of product characteristics. Takeda Manufacturing Austria AG; 2022. 20. Berinert 3000 IU. Summary of product characteristics. CSL Behring GmbH; 2021. 21. Watt M, Maurer M, Devercelli G, et al. Long-term impact of lanadelumab on patients with hereditary angioedema (HAE) type 1/2: patient-reported outcome (PRO) findings from the HELP open-label extension study (OLE). Poster presented at: American Academy of Asthma, Allergy & Immunology Virtual Annual Meeting; February 26-March 1, 2021. 22. Lumry WR, Maurer M, Weller K, et al; HELP OLE Study Group. Long-term lanadelumab treatment improves health-related quality of life in patients with hereditary angioedema. Ann Allergy Asthma Immunol. Published online April 5, 2023. doi:10.1016/j.anai.2023.03.028 23. Banerji A, Bernstein JA, Johnston DT, et al; HELP OLE Investigators. Long-term prevention of hereditary angioedema attacks with lanadelumab: the HELP OLE study. Supporting information. Figure S1. Allergy. 2022;77(3):979-990. Accessed February 16, 2023. https://onlinelibrary.wiley.com/doi/10.1111/all.15011 24. Banerji A, Riedl MA, Bernstein JA, et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. Supplement 1. Protocol and statistical analysis plan. JAMA. 2018;320(20):2108-2121. Accessed February 16, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6583584/bin/jama-320-2108-s001.pdf 25. Riedl MA, Bernstein JA, Craig T, et al. An open-label study to evaluate the long-term safety and efficacy of lanadelumab for prevention of attacks in hereditary angioedema: design of the HELP study extension. Clin Transl Allergy. 2017;7:36. doi:10.1186/s13601-017-0172-9